 |
A man in his 60s presents with progressive shortness of breath. |
- History of Present Illness
The patient was in his usual state of health until, three months prior to admission, he experienced a syncopal episode upon standing up from his bed in the morning. Over the next few days, he developed shortness of breath, lower extremity edema, and non-exertional, intermittent chest pain, causing him to present for evaluation to a primary care physician (PCP). He denied fevers, weight loss, night sweats, rash, headaches, vision changes, hearing loss, focal neurological changes, nausea, vomiting, diarrhea, or dysuria at time of presentation. He could recollect no previous illnesses within the preceding weeks nor any significant illnesses throughout his life. He was not taking any prescription, over-the-counter, or herbal medications. His PCP referred him to a cardiologist.
Two months prior to admission, the patient was seen by the cardiologist. Electrocardiogram (EKG) showed sinus rhythm with possible left atrial enlargement and no ST segment or T wave abnormalities. Transthoracic echocardiography (TTE) demonstrated an ejection fraction of 50%, moderate aortic valve stenosis and regurgitation, severe mitral stenosis and regurgitation, severe tricuspid regurgitation, and bi-atrial enlargement. He was diagnosed with presumed rheumatic heart disease and was prescribed diuretics. Despite this therapy, his symptoms worsened over the following 4 weeks.
Three weeks prior to admission, he presented to a local hospital for worsening dyspnea. Cardiac angiography revealed elevated right-sided pulmonary and left atrial pressures as well as 40% atherosclerotic disease of his left anterior descending coronary artery. Unfortunately, his social situation compelled him to leave that hospital before undergoing planned valve replacement surgery.
After discharge from the local hospital, our patient’s dyspnea continued to worsen, leading him to present to our hospital. He completed pre-operative evaluation and, on hospital day 17, underwent aortic and mitral valve replacement with mechanical valves and tricuspid valve repair via ring annuloplasty. The cardiothoracic surgery team described thickened, calcified aortic and mitral valves but a normal myocardium and aortic root.
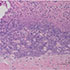
His initial post-operative course was unremarkable. However, on post-operative day (POD) 2, the Infectious Diseases service was consulted because the preliminary pathology report of the mitral valve tissue (which is routinely performed on all excised heart valves at our hospital) described necrosis with acute and chronic inflammatory cells consistent with abscess (Figure 1).
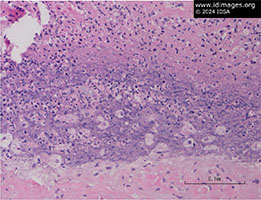 - Figure 1: Mitral valve anterior leaflets had few micro abscesses. No bacteria, fungi, or acid-fast bacteria are seen on gram, PAS, and AFB stains, respectively. Immunohistochemical stain for spirochetes did not show any bacteria.

- Past Medical History
- None
- Medications
- No antibiotics except for routine pre-operative prophylaxis with cefazolin on the day of procedure
- Furosemide 80 mg PO once daily
- Epidemiological History
The patient was born in Mexico and moved to the United States as a young adult. He worked in agriculture for a few decades throughout the United States. He reported several lifetime sexual partners, all women. His most recent sexual activity was about 10 months prior to admission. He had one sexual partner a few years before admission who was a commercial sex worker. He has no pets. He reported no outdoor hobbies.
- Physical Examination
- Before undergoing surgery for valve repair, the patient was afebrile, normotensive, with a normal heart rate, and on room air. At the time of the initial evaluation by the Infectious Diseases team on post-operative day 2, he was intubated, sedated, and intermittently moving in response to touch and voice. He was febrile to 101.1°F (38.4°C), pulse 76, ventilated with a PEEP of 5 and 40% FiO2, with blood pressures in the 90s/60s on epinephrine 2 mcg/min and milrinone 0.375 mcg/kg/min. He had a large midline sternotomy wound with minimal serosanguinous drainage and no surrounding erythema. He had chest tubes in place, distributed in the left and right pleural spaces. Chest auscultation revealed normal heart rate and rhythm with no murmurs as well as transmitted upper airway sounds related to the ventilator and decreased breath sounds in the bilateral lung bases. He had 1+ pitting edema bilaterally up to the mid-shins. Examination of his skin (including fingernails, finger pads, and palms) and joints were normal.
- Studies
- Pre-operative
• Complete blood count with differential was unremarkable
• Basic metabolic panel was unremarkable
• Liver function panel showed:
o AST 41 U/L (reference range: 13-39 U/L)
o ALT 56 U/L (reference range: 7-52 U/L)
o Bilirubin 1.5 mg/dL (reference range: 0.2-1.2 mg/dL)
Post-operative day 2 (day of consultation)
• Complete blood count without differential showed:
o WBC of 13,700 cells/uL (reference range: 4,500-12,000 cells/uL)
o Hemoglobin 7.6 g/dL (reference range: 14.0-18.0 g/dL)
o Platelets 88,000 cells/uL (reference range: 150,000-400,000 cells/uL)
• Basic metabolic panel was unremarkable
• Liver function panel showed:
o AST 69 U/L (reference range: 13-39 U/L)
o Bilirubin 2.5 mg/dL (reference range: 0.2-1.2 mg/dL)
Radiology/Imaging (pre-operative)
• TTE: ejection fraction 40-44%; moderate to severe aortic stenosis, moderate aortic valve regurgitation; severe mitral stenosis and regurgitation “appears rheumatic”; severe tricuspid regurgitation; left atrium severely dilated, right atrium severely dilated, right ventricle mildly dilated
• EKG: sinus rhythm, possible left atrial enlargement, no S-T segment or T wave abnormalities
• Chest x-ray: hazy airspace in the right lung base and enlarged cardiac silhouette.
• Computed tomography (CT) of the chest without contrast: moderate pulmonary edema with small right pleural effusion and anasarca suggestive of volume overload. Ground glass opacities in the bilateral, right greater than left lobes possibly suggestive of superimposed aspiration pneumonitis. Tracheomegaly measured to 3.7 cm above the aortic arch.
Preliminary Pathology
• Mitral valve: necrosis with acute and chronic inflammatory cells consistent with abscess (Figure 1) as well as focal collections of plasma cells (Figure 2) and foamy macrophages (Figure 3). Gram, periodic acid-Schiff (PAS), and AFB stains were negative. Immunohistochemical stains for spirochetes were negative.
• Aortic valve: hyaline degeneration and calcification
Tests for infectious diseases collected from peripheral specimens
• Pertinent negatives: Bartonella antibodies, Q-fever antibodies, Brucella antibodies, Rickettsia antibodies, urine Legionella antigen, QuantiFERON-TB Gold, serum and urine Histoplasma antigen, Trypanosoma cruzi IgG
• Negative 4th generation HIV antigen/antibody combination test, urine/oral/rectal gonorrhea/chlamydia nucleic acid amplification tests (NAAT), Hepatitis C IgG, Hepatitis B core antibody (IgG and IgM), Hepatitis B surface antigen
Microbiological
• Studies of the peripheral blood:
o 5 sets of routine aerobic/anaerobic cultures (3 collected pre-antibiotic administration) on two separate days were negative
o AFB cultures were negative
• Studies of the valve tissue: aerobic, anaerobic, AFB and fungal cultures negative
 - Figure 1: Mitral valve anterior leaflets had few micro abscesses. No bacteria, fungi, or acid-fast bacteria are seen on gram, PAS, and AFB stains, respectively. Immunohistochemical stain for spirochetes did not show any bacteria.

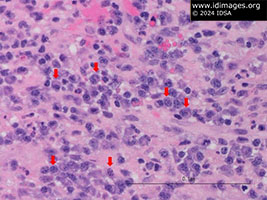 - Figure 2: Mitral valve anterior leaflets had focal collections of plasma cells (red arrows).

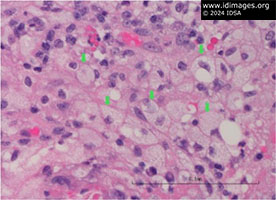 - Figure 3: Mitral valve anterior leaflets had foamy macrophages (green arrows).

- Clinical Course Prior to Diagnosis
Aortic and mitral valve tissue collected intra-operatively were sent for routine, anaerobic, fungal, and acid-fast bacillus (AFB) stains and cultures. On POD 2, three sets of blood cultures were collected, before the initiation of broad-spectrum antibiotics (vancomycin and cefepime).
Our patient recovered as expected post-operatively. On POD 6, he was extubated and all antimicrobials were stopped given negative cultures of the valve tissue and blood. His post-operative course was complicated by atrial flutter with rapid ventricular response, which was managed with amiodarone and metoprolol, as well as intermittent pleural bleeds, related to and managed with chest tubes, which were removed by POD 5.
- Treatment and Followup
The Treponema pallidum antibody (TPA) test was positive as was the reflexive reactive plasma reagin (RPR) test (titer 1:32). In the setting of no prior knowledge of syphilis (confirmed by both the patient and the local health department), he was given one dose of 2.4 million units of intramuscular (IM) benzathine penicillin G, and two additional weekly doses were planned for late, latent syphilis. The remaining serologic workup was negative, as described above. Next generation sequencing (NGS) of the mitral valve tissue was positive for Treponema pallidum (NGS coverage 59%) and Bacillus licheniformis (NGS coverage 28%).
Given the unclear timeline of history and stage of syphilis (with concern for tertiary syphilis), we planned to pursue a lumbar puncture to rule out neurosyphilis. However, our patient was unable to undergo lumbar puncture due to his elevated INR related to therapeutic warfarin required to protect his mechanical heart valves. Thus, rather than simply completing the 3 weekly doses of IM benzathine penicillin G recommended for tertiary syphilis, we treated with 10 days of intravenous (IV) aqueous crystalline penicillin G 4 million units every 4 hours. Treatment with IV penicillin began after the patient had already received two IM injections of benzathine penicillin G and therefore adequately treated tertiary syphilis as well. After receiving a pacemaker for frequent post-operative bradycardia, he was discharged on post-operative day 21 and remained in good health at clinic follow up several months later. - Discussion
- Cardiac manifestations of syphilis
Treponema pallidum is the causative agent of syphilis. It may be transmitted sexually or from mother-to-child. Historically, syphilis is classified based on duration of infection and symptoms into the following categories: primary, secondary, early latent, late latent, tertiary, and neurosyphilis. Twenty-five percent of untreated people with T. pallidum infection will develop tertiary syphilis1 - a term denoting symptomatic late syphilis. The cardiac manifestations of tertiary syphilis are feared because of their significant associated morbidity and mortality. Classically, the pathology of cardiac syphilis is related to dissemination of T. pallidum via the aortic vasa vasorum, leading to inflammation (i.e., vasculitis) and compromise of the blood supply of the aortic wall. Broadly, five categories of cardiovascular syphilis are described.2 First, uncomplicated syphilitic aortitis, which occurs at time of primary infection in 70-80% of patients and typically involves the transverse aorta and aortic arch. Second, aortic aneurysm, where endarteritis of the vasa vasorum results in fibrous replacement of the elastic tissue of the aortic wall, weakening it and allowing for “ballooning.”3 Third, aortic insufficiency, due to aortic root enlargement dilating the aortic valve annulus and widening the commissures. Fourth, coronary ostial stenosis, which occurs in 29% of cases of syphilitic aortitis when inflammation extends to the coronary ostium.4 Fifth, syphilitic myocarditis, which was described by the well-known pathologist Dr. Warthin as a common end-stage complication of syphilis with a spectrum of manifestations ranging from “microscopic areas of...plasma cell and lymphocyte infiltrations up to more diffuse areas of interstitial myocarditis.”5 Regarding manifestations of cardiac syphilis outside of these historic categories, several reports of endocarditis of the aortic valve attributed to T. pallidum infection are published.6,7 From our literature review, it appears that our case is the first report of T. pallidum infection of the mitral valve confirmed by molecular testing, suggesting that T. pallidum may cause additional types of cardiac disease that have not yet been well-described.
Diagnosis of syphilis
As T. pallidum cannot be grown in culture, direct (e.g., dark field microscopy, histological examination of tissues, and molecular) and indirect (e.g., serologic) methods of detecting T. pallidum are utilized for syphilis diagnosis. The original gold standard of syphilis diagnosis was the rabbit infectivity test (first used for T. pallidum in 1907).8 In modern times, improved indirect treponemal tests (first developed 1949) and nontreponemal (e.g., rapid plasma reagin [RPR], developed in 1957) are used. These tests are reliable for syphilis diagnosis if used as a two-step algorithm, which should include each of the following:
• A treponemal test like T. pallidum particle agglutination (TP-PA), which detects antibodies to Treponema species and is highly sensitive (94.5-96.4%) and specific (99.0-100.0%) but does not differentiate between past and current T. pallidum infection.9
• A non-treponemal test like RPR, which detects antibodies to lipoidal antigens and is considered less specific (81-96%) but is reported in titers and can therefore be used to assess treatment response.10 Notably, non-treponemal tests may be negative in up to 30% of tertiary syphilis cases.11
Molecular tests such as nucleic acid amplification (NAAT) and next generation sequencing (NGS) are newer methods for the diagnosis of syphilis, although their cost and complexity prohibit common use and widespread availability. NAATs are invariably highly specific (98-100%) but with variable sensitivity by specimen source, stage of disease, and polymerase chain reaction (PCR) target (0-95%).12 In latent syphilis, detection ranged from 0-44%.12-14 Data for NGS are limited; some reports describe the utility of NGS for diagnosis of early-stage syphilis.15 NGS has proven useful in other cases of culture-negative endocarditis, with reports of 85.9-100% sensitivity and a positive predictive value of 97% when testing heart valve tissue.16 Molecular testing can be a useful tool for diagnosis of challenging cases, particularly with organisms that are difficult to culture or when little tissue is available, such as in the case presented here. In our case, the diagnosis of mitral valve endocarditis due to T. pallidum is supported by (1) the atypical endocarditis presentation (effectively ruling out pathogens like Staphylococcus aureus), (2) extensive negative workup (e.g., blood and valve cultures and serologic tests for other organisms known to culture-negative endocarditis), (3) pathology definitively showing findings consistent with bacterial infection, (4) T. pallidum being the only organism present (via RPR and NGS), and (5) NGS validity in endocarditis diagnosis with high positive predictive values.
Rheumatic heart disease and Treponema pallidum infection
Rheumatic fever is caused by the host immune response to untreated group A Streptococcus infection, which can cause damage and scarring of the heart valves, leading to a host of cardiac complications including aortic, mitral, and (less commonly) tricuspid valve stenosis and regurgitation, years to decades after initial infection. Rheumatic heart disease increases the patient’s risk of developing infective endocarditis; 15-55% of endocarditis cases are reported to occur in patients with rheumatic heart disease in countries reporting higher rheumatic heart disease prevalence.17-18 Heart valves damaged by rheumatic fever provide an ideal surface on which circulating bacteria can adhere to and form vegetations. No robust studies have investigated the interaction between T. pallidum and rheumatic heart disease. Authors of one pre-antibiotic era study of 6 cases with both rheumatic and syphilitic cardiovascular disease hypothesized that T. pallidum infection may exacerbate underlying, asymptomatic rheumatic heart disease,7 but little data are available to support this theory. Rather than “exacerbating” or “re-activating” pre-existing rheumatic heart disease, we suspect that T. pallidum may utilize the damaged heart valve as a nidus of infection, as can other indolent bacterial agents of culture-negative endocarditis. Data to support these theories are sparse as tertiary syphilis is relatively rare due to widespread availability and use of antibiotics. However, syphilis rates are high,19 so we may see more tertiary syphilis in the future.
- Final Diagnosis
- Mitral valve endocarditis due to Treponema pallidum
- References
-
- Gjestland, T. (1955). The Oslo study of untreated syphilis, an epidemiologic investigation of the natural course of syphilitic infection based upon a restudy of the Boeck-Bruusgaard material. Acta Derm Venereol, 35, S-34.
PMID:13301322 (PubMed abstract)
- Duncan, J. M., & Cooley, D. A. (1983). Surgical considerations in aortitis: part III: syphilitic and other forms of aortitis. Texas Heart Institute Journal, 10(4), 337.
PMID:15226966 (PubMed abstract)
- Berhil, T., Radi, F. Z., El Boussaadani, B., & Raissouni, Z. (2024). Tertiary syphilis and cardiovascular disease: the united triad: case report. European Heart Journal-Case Reports, 8(3), ytae013.
PMID:38476287 (PubMed abstract)
- Heggtveit, H. A. (1964). Syphilitic aortitis: a clinicopathologic autopsy study of 100 cases, 1950 to 1960. Circulation, 29(3), 346-355.
PMID:14128825 (PubMed abstract)
- Warthin, A. S. (1929). Lesions of latent syphilis. British Medical Journal, 2(3579), 236.
PMID:20774845 (PubMed abstract)
- Hijikata, S., Hongo, I., Nakayama, S. I., Yamaguchi, T., Sekikawa, Y., Nozato, T., & Ashikaga, T. (2019). Infective endocarditis due to treponema pallidum: A case diagnosed using polymerase chain reaction analysis of aortic valve. Canadian Journal of Cardiology, 35(1), 104-e9.
PMID:30595174 (PubMed abstract)
- Lisa, J. R., & Chandlee, G. J. (1934). The heart and great vessels in combined syphilitic and rheumatic infection. Archives of Internal Medicine, 54(6), 952-980.
- Magnuson, H. J., Hill, C., Eagle, H., & Fleischman, R. (1948). The minimal infectious inoculum of Spirochaeta pallida (Nichols strain), and a consideration of its rate of multiplication in vivo.
PMID:24396138 (PubMed abstract)
- Park, I. U., Fakile, Y. F., Chow, J. M., Gustafson, K. J., Jost, H., Schapiro, J. M., ... & Bolan, G. (2019). Performance of treponemal tests for the diagnosis of syphilis. Clinical Infectious Diseases, 68(6), 913-918.
PMID:29986091 (PubMed abstract)
- Sato, I., Nakamachi, Y., Ohji, G., Yano, Y., & Saegusa, J. (2022). Comparison of 17 serological treponemal and nontreponemal assays for syphilis: A retrospective cohort study. Practical Laboratory Medicine, 32, e00302.
PMID:36217361 (PubMed abstract)
- Ratnam, S. (2005). The laboratory diagnosis of syphilis. Canadian Journal of Infectious Diseases and Medical Microbiology, 16, 45-51.
PMID:18159528 (PubMed abstract)
- Theel, E. S., Katz, S. S., & Pillay, A. (2020). Molecular and direct detection tests for Treponema pallidum subspecies pallidum: a review of the literature, 1964–2017. Clinical Infectious Diseases, 71(Supplement_1), S4-S12.
PMID:32578865 (PubMed abstract)
- Gayet-Ageron, A., Ninet, B., Toutous-Trellu, L., Lautenschlager, S., Furrer, H., Piguet, V., ... & Hirschel, B. (2009). Assessment of a real-time PCR test to diagnose syphilis from diverse biological samples. Sexually transmitted infections, 85(4), 264-269.
PMID:19155240 (PubMed abstract)
- Castro, R., Prieto, E., Aguas, M. J., Manata, M. J., Botas, J., Santo, I., ... & Pereira, F. L. H. (2007). Detection of Treponema pallidum sp pallidum DNA in latent syphilis. International journal of STD & AIDS, 18(12), 842-845.
PMID:18073019 (PubMed abstract)
- Cheng, W., Liu, J., Mou, Y., Ji, C., Ren, H., & Hu, W. (2023). Next-generation sequencing for diagnosis and prognosis in early-stage syphilis. International Journal of Dermatology, 62(11), 1428-1428.
PMID:37753819 (PubMed abstract)
- Haddad, S. F., DeSimone, D. C., Chesdachai, S., Gerberi, D. J., & Baddour, L. M. (2022). Utility of metagenomic next-generation sequencing in infective endocarditis: a systematic review. Antibiotics, 11(12), 1798.
PMID:36551455 (PubMed abstract)
- Xu, H., Cai, S., & Dai, H. (2016). Characteristics of infective endocarditis in a tertiary hospital in East China. PloS one, 11(11), e0166764.
PMID:27861628 (PubMed abstract)
- Kucukates, E., Gultekin, N., & Bagdatli, Y. (2013). Cases of active infective endocarditis in a university hospital during a 10-year period. Hypertension, 6, 27-27.
PMID:24601198 (PubMed abstract)
- CDC. (2022). Sexually Transmitted Infections Surveillance. https://www.cdc.gov/std/statistics/2022/default.htm
- Notes
- ID Week 2024 - Fellows' Day
Tyler Brehm, MD
PGY-5, Infectious Diseases Fellow
Baylor College of Medicine, Houston, TX
Acknowledgements
Baylor College of Medicine Division of Infectious Diseases: Eva Clark, MD, PhD and Jill Weatherhead, MD, PhD
Baylor College of Medicine Division of Pathology and Immunology: Cameron Brown, PhD and Neda Zarrin-Khameh, MD
Baylor College of Medicine Division of Cardiothoracic Surgery: Ravi Ghanta, MD and Christopher Sylvester, MD, PhD
- Citation
- If you refer to this case in a publication, presentation, or teaching resource, we recommend you use the following citation, in addition to citing all specific contributors noted in the case:
Case #24001: A man in his 60s presents with progressive shortness of breath. [Internet]. Partners Infectious Disease Images. Available from: http://www.idimages.org/idreview/case/caseid=612
- Other Resources
-
Healthcare professionals are advised to seek other sources of medical information in addition to this site when making individual patient care decisions, as this site is unable to provide information which can fully address the medical issues of all individuals.
|
|