 |
New Fever and Consolidation in a patient recovering from Covid-19 |
- History of Present Illness
A man in his 50s presented with dry cough, intermittent fever, progressive dyspnea on exertion and fatigue of 1-week duration. One day prior to presentation he also developed non-bloody diarrhea without abdominal cramping, pain, nausea and vomiting. He was diagnosed with Covid-19 five days prior to presentation by a nasopharyngeal viral PCR that was positive for SARS-CoV-2. A chest radiograph revealed patchy infiltrates in the bilateral perihilar region and bases, right greater than left [Fig 1]. He was admitted for supportive care and monitoring. On day 5 of hospitalization he developed progressive hypoxemic respiratory failure requiring mechanical ventilation. A follow up chest radiograph revealed worsening infiltrates bilaterally. He was given tocilizumab intravenously at 600 milligram every 8 hours for a total of 3 doses and 200 milliliters of convalescent plasma. His sputum culture obtained at the time of intubation grew methicillin sensitive Staphylococcus aureus for which intravenous cefazolin was started. After extubation on hospitalization day 14, he developed a new fever and elevated white blood cell count.
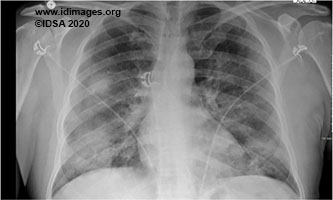 - Figure 1. Chest Radiograph single view shows patchy infiltrates in the bilateral perihilar region and bases, right greater than left

- Past Medical History
Diabetes Mellitus Type 2. Hypertension
- Medications
Metformin, Lisinopril.
- Epidemiological History
Patient’s wife and children also recently had Covid-19. No pets at home and no recent travel.
- Physical Examination
Patient appeared pale, anxious and was in mild respiratory distress. He had a temperature of 38.5C [101.3F]. His respiratory rate was 28 per minute, pulse 110 per minute, blood pressure 121/65 mm Hg and saturating 96% on 4 liters of oxygen. Chest examination revealed bilateral rales with no accessory muscle use. Examination otherwise unremarkable.
- Studies
White blood cell count of 25,010 cells/microliter (reference range 4000 – 10800 cells/microliter) and absolute neutrophils 15,780 cells/microliter (reference range 1800 – 7800 cells/microliter). His platelet count was 282,000 cells/microliter (reference range 140,000 – 400,000 cells/microliter). His serum creatinine was 0.79 milligram/deciliter (reference range 0.60 – 1.30 milligram/deciliter). His hemoglobin A1C was 9.9% (reference range 4.1% – 5.6%). Liver function tests were normal. A CT angiography of thorax revealed peripheral ground glass opacification as well as more dense consolidation in the superior segments of bilateral lower lobes with bubbly appearance on the right concerning for necrotizing pneumonia [Fig 2]. A sputum culture was obtained.
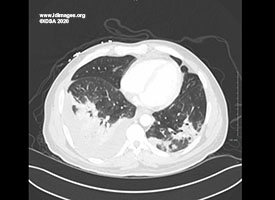 - Figure 2. CT Angiography of the Thorax shows dense consolidation in the superior segments of bilateral lower lobes with bubbly appearance on the right concerning for necrotizing pneumonia

- Diagnostic Procedure(s) and Result(s)
He was continued on cefazolin with no significant change in clinical status. Two days later his sputum culture grew the organism shown in lactophenol cotton blue stain [Fig 3].
The sputum culture grew Aspergillus fumigatus. Serum Aspergillus galactomannan antigen resulted with an index value of greater than 1.110 (reference range >0.5 – patients with an index value of greater than or equal to 0.5 are considered to be positive for galactomannan antigen). Beta-D Glucan was negative at less than 31 picogram/milliliter (reference range <80 picogram/milliliter). Histoplasma and Blastomyces serologies were negative. A bronchoscopy was deferred due to patient’s tenuous respiratory status. A diagnosis of probable invasive pulmonary aspergillosis was made based on CT findings, microbiological data and positive galactomannan antigen.
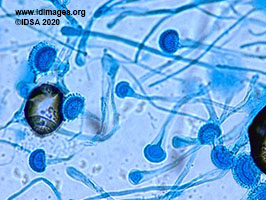 - Figure 3. Lactophenol cotton blue stain from sputum culture shows Aspergillus fumigatus

- Treatment and Followup
He was started on oral voriconazole with initial loading dose of 400 milligram twice a day followed by maintenance dosing of 200 milligram twice a day. Voriconazole is planned to be continued for a total of 12 weeks. He was discharged to a rehabilitation facility and slowly improved. He remained afebrile and was completely weaned off of oxygen at the time of discharge.
- Discussion
Critically ill patients with Covid-19 have a high risk of secondary infections. Invasive pulmonary aspergillosis (IPA) is increasingly reported as a co-infection in people with Covid-19 pneumonia (1,3). IPA is a well described complication of severe influenza (1,2), though the exact rate of IPA in Covid-19 patients is yet to be known; in some series, the rate is 30%, similar to influenza (2). In a study from France, 33% out of 27 patients was found to have putative IPA (4) and Koehler et al have published a study from Germany that shows a prevalence of 26% out of 19 critically ill patients (1).
The highly aggressive nature of SARS CoV-2 causing bilateral alveolo-interstitial lesions increases the risk of infections acquired through inhalational route specifically aspergillosis. Less ciliary clearance, elevated IL-6 levels and IL-1 pathway activation promoting fungal growth are possible factors in Covid-19 associated IPA. Immune dysregulation associated with Covid-19 affecting Th1 and Th2 responses could be a risk factor for IPA (5).
Our patient had no host risk factors similar to the other reported cases of Covid-19 with IPA. There is a high risk of missed diagnosis if the diagnosis of probable or proven IPA is made based on the existing consensus definitions like European Organization for the Research and Treatment of Cancer/Mycoses Study Group EORTC/MSG and AspICU algorithm.
Diagnosis of IPA by these definitions require a positive histopathology for invasive disease, typical host risk factors, Aspergillus growth in lower respiratory tract culture and/or positive biomarker assays for Aspergillus antigen. Absence of host factors was also apparent in influenza-associated disease, in which 57% of patients could not be classified based on AspICU algorithm for patients in ICUs. A retrospective multicenter cohort study showed that influenza infection was an independent risk factor for invasive pulmonary aspergillosis (2). Diagnosis of IPA is crucial and bronchoalveolar lavage (BAL) has an important diagnostic role. However, bronchoscopy and BAL in Covid-19 are limited due to risk of aerosolization. Serial measurements of fungal markers including serum galactomannan and serum beta D-glucan can be valuable in the diagnosis along with radiographic and microbiological data from sputum, endotracheal aspirate and/or BAL cultures.
Since mortality rates are higher in untreated IPA, treatment is warranted in patients with high suspicion for IPA. Voriconazole is the first line antifungal for IPA and patients should be treated for a total of 12 weeks with adequate follow up with serum galactomannan and imaging. Until we gain crucial insights into IPA and Covid-19 lung, patients with Covid-19 who are critically ill with evidence for Aspergillus infection should receive antifungal therapy to prevent increased mortality (5).
- Final Diagnosis
Probable Invasive Pulmonary Aspergillosis in a Person with Covid-19
- References
-
- Phillip Koehler et al., Covid-19 Associated Pulmonary Aspergillosis. Mycoses 2020 Jun;63(6):528-534.
PMID:32339350 (PubMed abstract)
- Schauwvlieghe AFAD, Rijnders BJA, Philips N, Verwijs R, Vanderbeke L, Van Tienen C, et al.; Dutch-Belgian Mycosis study group. Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: a retrospective cohort study. Lancet Respir Med. 2018; 6:782–92
PMID:30076119 (PubMed abstract)
- Wauters J, Baar I, Meersseman P, et al. Invasive pulmonary aspergillosis is a frequent complication of critically ill H1N1 patients: a retrospective study. Intens Care Med 2012; 38: 1761–8.
PMID:22895826 (PubMed abstract)
- Alanio A, Dellière S, Fodil S, Bretagne S, Mégarbane B. Prevalence of putative invasive pulmonary aspergillosis in critically ill patients with COVID-19. Lancet Respir Med. 2020 Jun;8(6):e48-e49. doi: 10.1016/S2213-2600(20)30237-X. Epub 2020 May 20.
PMID:32445626 (PubMed abstract)
- Paul E Verweij, Jean-Pierre Gangneux, Matteo Bassetti, Roger J M Bru¨ggemann, Oliver A Cornely, Philipp Koehler, Cornelia Lass-Flo¨rl, Frank L van de Veerdonk, Arunaloke Chakrabarti, Martin Hoenigl, Diagnosing Covid-19 associated Pulmonary Aspergillosis., On behalf of the European Confederation of Medical Mycology,the International Society for Human and Animal Mycology, the European Society for Clinical Microbiology and Infectious Diseases Fungal Infection Study Group, and the ESCMID Study Group for Infections in Critically Ill Patients Diagnosing Covid-19 associated pulmonary aspergillosis Lancet Microbe 2020 Published Online May 8, 2020 https://doi.org/10.1016/ S2666-5247(20)30027-6.
PMID:32835328 (PubMed abstract)
- Notes
ID Week Fellows' Day 2020- oral presentation
This case was contributed by:
Vetriselvi Moorthy, MD
Spectrum Health/Michigan State University, Grand Rapids, MI
The case was originally presented at ID Week 2020, a joint effort of Infectious Diseases Society of America (IDSA), HIV Medical Association, Pediatric Infectious Diseases Society (PIDS), and the Society for Healthcare Epidemiology of America (SHEA), during an interactive session on Fellows' Day. Copyright Infectious Disease Society of America (IDSA), 2020. Used with permission.
- Citation
- If you refer to this case in a publication, presentation, or teaching resource, we recommend you use the following citation, in addition to citing all specific contributors noted in the case:
Case #20002: New Fever and Consolidation in a patient recovering from Covid-19 [Internet]. Partners Infectious Disease Images. Available from: http://www.idimages.org/idreview/case/caseid=584
- Other Resources
-
Healthcare professionals are advised to seek other sources of medical information in addition to this site when making individual patient care decisions, as this site is unable to provide information which can fully address the medical issues of all individuals.
|
|